Difference between physical adsorption and chemical adsorption - Database & Sql Blog Articles
The adsorption mechanism of gas molecules on solid surfaces is extremely complex, including physical adsorption and chemical adsorption. ? Cc/g?@STP ? indicates. ? At the low temperature, physical adsorption is dominant, and possible chemical adsorption occurs at high temperatures (specific reactions occur). The whole process involves high vacuum, low temperature, high temperature, high precision vacuum measurement, and the valve is set according to the preset The program automatically switches and other issues. Ningbo Autrends International Trade Co.,Ltd. , https://www.supervapebar.com
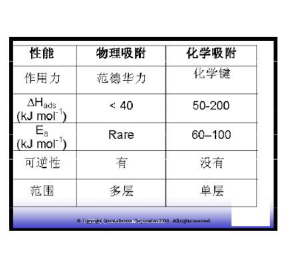
The adsorption produced by the intermolecular force (van der Waals force) is called physical adsorption. Physical adsorption is a common phenomenon that exists on the surface of a solid (adsorbent) that is brought into contact with the adsorbed gas (adsorbed material). The intermolecular forces involved are of the same type, for example, causing defects in the actual gas and condensation of the vapor. In addition to attracting dispersive forces and repulsive forces at close range, specific intermolecular interactions (eg, polarization, field-dipole, field) typically occur due to the specific geometry of the adsorbent and adsorbent species and the electronic properties of the outer layer. The quadrupole moment of the gradient). ? Any interaction between molecules, so physical adsorption is not selective, activation energy is small, adsorption is easy, and desorption is also easy. It can be monolayer adsorption and multi-layer adsorption. ?
The adsorption caused by the formation of chemical bonds between molecules is called chemical adsorption; it is selective, the activation energy is large, the adsorption is difficult, and the desorption is difficult, and a higher temperature is often required. Chemical adsorption must be monolayer adsorption. Actual adsorption may have both physical adsorption and chemical adsorption; first physical adsorption followed by chemical adsorption. The amount of adsorption can be measured by the volume of the adsorbed substance (adsorbed matter) per unit mass of sample (adsorbent) at standard atmospheric pressure. It can be used in ml/g? or?