China's electric vehicle battery technology research and development and market status
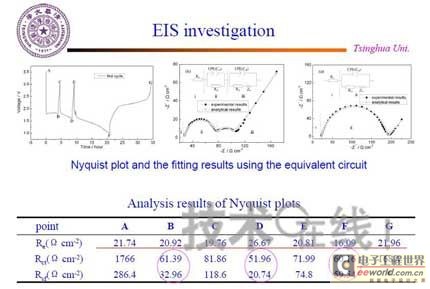
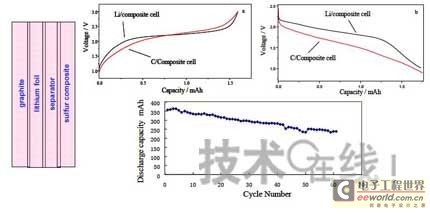
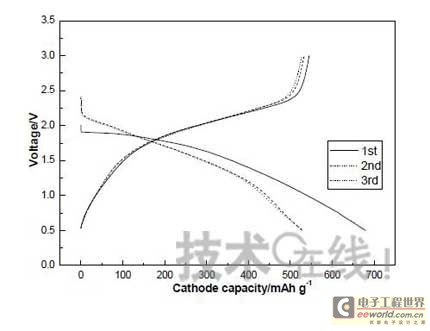
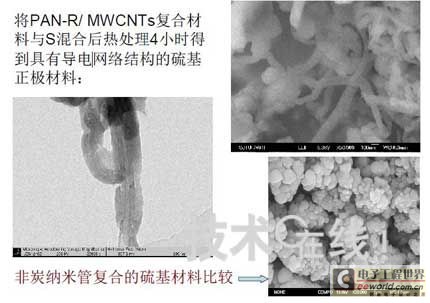
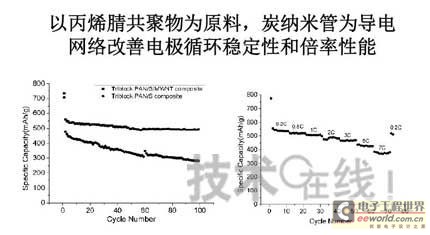
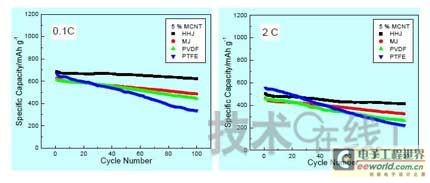
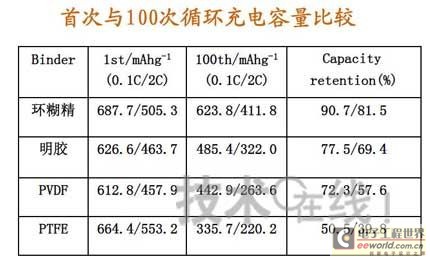
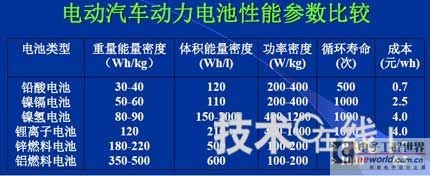
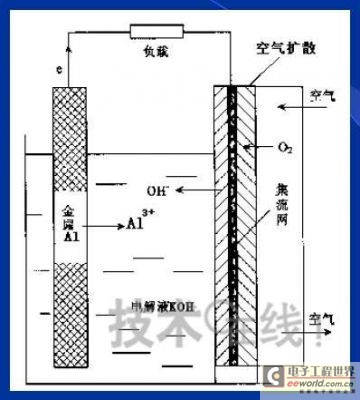
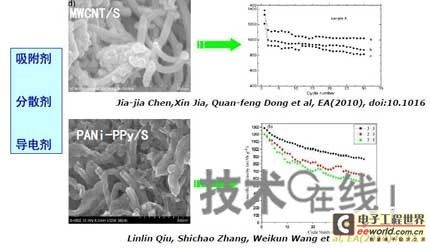
Research institutions around the world are stepping up research on new energy batteries for future market demand, such as lithium-sulfur batteries, metal (lithium, aluminum, zinc) air batteries. The characteristics of such batteries are low raw material cost, low energy consumption, low toxicity and high energy density. Lithium-sulfur batteries have an energy density of 2,600 Wh/kg, and lithium-air batteries have an energy density of 3,500 Wh/kg. This article refers to the address: http:// Lithium-sulfur battery Lithium-sulfur battery has become one of the research directions of Japan's new energy vehicle power battery technology. The Japan New Energy Industry Technology Development Organization (NEDO) has invested 30 billion yen (about 2.4 billion yuan) in research and development budget every year since 2009. The goal is to achieve an energy density of 500 Wh/kg by 2020. The United States has moved faster in this regard, and its Ministry of Energy recently invested $5 million to fund research on lithium-sulfur batteries, with an energy density of 500 Wh/kg planned for 2013. Representative manufacturers of lithium-sulfur battery research in the world include Sion Power, Polyplus, Moltech in the United States, Oxis in the United Kingdom, and Samsung in South Korea. Polyplus' 2.1Ah lithium-sulfur battery has an energy density of 420Wh/kg or 520Wh/l. In July 2010, Sion Power's lithium-sulfur battery performance for the US drone power source was striking. The drone was charged by solar cells during the day and powered by night discharge, creating a record of 14 days of continuous flight. The near-term goals for energy density and cycle performance are over 500 Wh/kg and 500 cycles, respectively. By 2016, it will reach 600Wh/kg and 1000 cycles. In China, Tianjin Electronics 18, Chemical Research Institute, Tsinghua University, Shanghai Jiaotong University, National University of Defense Technology, Wuhan University, Beijing Institute of Technology, etc. are conducting research on lithium-sulfur batteries. It was found that the cycle stability of lithium-sulfur batteries was caused by the discharge dissolution of the positive active material and the instability of the surface of the metallic lithium, the electrical insulation of the sulfur itself and its discharge products (5x10-30S/cm). Poor, the utilization of active materials is low. Large mesoporous carbon cathode material The cathode material of lithium-sulfur battery includes porous carbon, such as large mesoporous carbon, activated carbon, carbon gel, etc. (see Table 1); carbon nanotubes, nanostructured conductive polymer materials such as MWCNT, PPy, PANi/PPy, etc. (see Figure 1); and PAN. Table 1 Electrochemical properties of porous carbon/sulfur composites with different pore structures Figure 1 Carbon nanotubes and nanostructured polymer sulfur composites Dr. Wang Weikun of the China Institute of Chemical Research said at the "Future Electric Vehicle High Energy Power Seminar" held at Fudan University in Shanghai on September 16 that large mesoporous carbon can form parasitic carbon-sulfur complexes by filling elemental sulfur. Use high pore volume of carbon (>1.5cm3/g) to ensure high filling capacity of sulfur to achieve high capacity; use high carbon surface density (>500cm2/g) to adsorb discharge products, improve cycle stability; utilize high conductivity of carbon (Several S/cm) Improves the electrical insulation of elemental sulfur, improves the utilization of sulfur and the charge and discharge rate performance of the battery. Figure 2 Preparation of large mesoporous carbon-sulfur composite Since the low voltage platform of the sulfur electrode is closely related to the viscosity of the electrolyte, the higher the viscosity, the lower the low voltage platform; the higher the ratio of conductivity to viscosity, the better the electrochemical performance of the battery. Therefore, the optimum composition of the electrolyte is 0.65 M LiTFSI/DOL + DME (volume ratio 1:2). Gelatin adhesive has good adhesion and dispersibility. It does not dissolve or swell in the electrolyte of lithium-sulfur battery. It can promote the complete oxidation of polysulfide ions into elemental sulfur during charging, which can improve the discharge capacity of lithium-sulfur batteries. Cycle performance. The porous electrode is prepared by a "freeze drying, ice crystal hole making" process, which can ensure the deep infiltration of the electrolyte and reduce the loss of the active reaction site due to the coverage of the discharge product. The energy density of the 1.7Ah lithium-sulfur battery of the Institute of Chemical Defense is 320 Wh/kg; under 100% DOD discharge, the cycle is 100 times, the capacity retention rate is about 75%, and the cycle efficiency is up to 70%. The self-discharge rate in the first year is about 25%, the average monthly self-discharge rate is 2~2.5%; the 0°C discharge capacity reaches more than 90% of the normal temperature capacity, and the tolerance at -20°C is 40% of the normal temperature capacity; When the battery is discharged/overcharged, the battery is incombustible and does not explode. When the battery is overcharged, the battery bulges and bubbles are generated inside. Wang Weikun said that in the future, he is preparing to strengthen the research on lithium metal anodes. On the one hand, it is necessary to stabilize the surface and prevent the occurrence of dendrites. The surface should be improved in its high current discharge capacity to enhance the rate discharge performance of lithium-sulfur batteries. Vulcanized polypropylene (SPAN) cathode material Professor He Xiangming of Tsinghua University has developed a polymer lithium battery with a propylene sulfide (SPAN) as a positive electrode material and a capacity of 800 mAh/g. The energy density of the lithium/vulcanized polypropylene clear battery exceeds 240 Wh/kg. Vulcanized polypropylene materials have ultra low cost and low energy consumption. In addition, graphite / vulcanized polypropylene batteries will become a strong candidate for large lithium batteries. A lithium secondary battery based on a reversible electrochemical reaction can be a conductive polymer by doping and dedoping sulfur, and vulcanizing the pyrolyzed polypropylene. The capacity of the vulcanized polypropylene battery is larger than that of the lithium battery based on the reversible electrochemical reaction. The special charge and discharge characteristics indicate that the sulfide battery far exceeds the lithium battery mechanism. He Xiangming's research results show that when the deep discharge reaches 0V, the discharge/charge capacity is 1502mAh/g and 1271mAh/g, and then the cycle is stable between 1V and 3V. The cycle performance is stable between 0.1 V and 3 V, and the capacity is 1000 mAh/g. For overcharging, the voltage suddenly drops to 3.88V, and then stabilizes at around 2V. After overcharging, the charging cannot be continued, indicating that the battery has the inherent safety of overcharging. The upper limit voltage for charging is 3.6V. When the charging voltage reaches 3.8V, it can no longer continue charging; when the voltage reaches 3.7V, it cannot be recharged after 3 cycles. In addition, the two sulfide/lithium batteries have almost the same discharge voltage as the two lithium cobaltate/lithium batteries, and therefore, they have good interchangeability. The charging voltage and capacity of such a battery increase as the temperature decreases. The discharge capacities at 60 ° C and -20 ° C were 854 and 632 mAh/g, respectively. The polymer anode has an operating temperature above -20 °C. The charging voltage and capacity will decrease as the current density increases. At a current density of 55.6 mA/g, the capacity is 792 mAh/g; when the current density is 667 mA/g, the capacity is 604 mAh/g. This indicates that the battery can operate in a state where the current density is high. The sulfide electrode expands in volume when discharged (intercalated with lithium ions) and shrinks when charged (de-lithium ions) (see Table 2). After the first discharge, the positive electrode thickness increases by about 22%. The thickness variations of the metal lithium negative electrode and the sulfide positive electrode compensate each other to ensure that the overall thickness of the battery does not change too much. Conductive polymers also have the same properties. In the EIS study, the results of the measurement and fitting of the equivalent circuit are shown in Fig. 3. Table 2 Changes in polymer electrode thickness Figure 3 Measurement and fitting results of the equivalent circuit Due to the different structure of the vulcanized pyrolyzed polypropylene (SPAN) and pyrolytic polypropylene (PPAN), the former can remain stable above 600 °C. A prototype polymer lithium battery using a sulfided polypropylene as a positive electrode and a lithium foil as a negative electrode has a size of 4x40x26 mm3 and an energy density of 246 Wh/kg or 401 Wh/l. In addition, in the experiment of using graphite as the negative electrode of lithium-sulfur battery, in a dry air or inert gas box, Celgard's 2400-hole diaphragm is used as a separator, which is placed between the positive and negative electrodes to form a cell, and the negative electrode and the separator are separated. Between the sheets is a 100 μm thick lithium foil material, which is then filled with 1M LiPF6-EC/DEC electrolyte and finally sealed into a button cell. The characteristic curve is shown in Figure 4. The charge and discharge curves after adding Li2.6Co0.4N are shown in Fig. 5. Fig. 4 Characteristic curve of cathode as lithium-sulfur polymer battery with graphite Figure 5 Charge and discharge curves after adding Li2.6Co0.4N Among the above two methods, it is safer to use graphite as the negative electrode than metal lithium; the sulfide positive electrode before lithiation is formed by electrochemical lithiation; there is a voltage of 0.2V between the sulfide/graphite battery and the sulfide/lithium battery. Poor; sulfide/graphite batteries have a more stable cycle life. Carbon nanotube vulcanized polyacrylonitrile cathode material Another research result on sulfur-based composite cathode materials is the sulfur-containing composite cathode material of polyacrylonitrile copolymer grown on the surface of carbon nanotubes studied by Professor Yang Jun of the School of Chemistry and Chemical Engineering, Shanghai Jiaotong University (see Figure 6). This is a sintered product of type B polyacrylonitrile, sulfur and 5% carbon nanotubes. MWCNTs with a diameter of about 20 nm penetrate between the particles, reducing the size of the secondary particles, forming a good structural skeleton and a conductive network. As the carbon tube content increases, the initial capacity decreases, but the cycle stability and rate performance of the electrode are improved (see Figure 7). Figure 6 Sulfur-based cathode material with conductive network structure Figure 7 increases the carbon tube content, the initial capacity is reduced, but the cycle stability and rate performance of the electrode are improved. Cyclodextrin is used as an electrode binder because it has the best cycle performance at both small current and high current rates. Figure 8 and Table 3 compare the effects of several electrode binders on battery performance. (Reporter En Ping) Figure 8 Comparison of the effects of several electrode binders on battery performance Table 3 Comparison with the first 100 cycles of charging capacity Metal air battery At present, the 330V/60Ah battery pack of lithium iron phosphate battery used in the BYD F3 dual-mode electric vehicle on the market has only 19.8kWh and weighs 230kg. The actual energy density is only 86Wh/kg. If this battery is used up to 60kWh (about driving 400 km), the weight will reach an unacceptable 700kg. In addition, the electric buses produced in China all claim to have a cruising range of up to 300 kilometers, but the pure electric buses on the World Expo use 3600kg batteries (12 pieces each, 300kg each) can only travel 110~120km without air conditioning. If you turn on the air conditioner, you can only continue to drive for 80 kilometers, and the average daily operating mileage of the bus is 250 kilometers. Due to the safety of the battery, it is impossible to charge and discharge deeply. Therefore, the actual available energy is less than half the nominal energy of the battery. Yang Deqian, chief designer of China PowerSource (Powerzinc), pointed out the shortage of existing power batteries in the Chinese market with the above two examples in the "Future Electric Vehicle High Energy Power Seminar". Tang Yougen, director of the Institute of Chemical Power and Materials at Central South University, agreed with Yang Deqian's point of view. He used a set of data to specify the advantages of metal-air batteries compared to existing power batteries (see Table 4). Table 4 Comparison of performance parameters of electric vehicle power battery In China, metal and air batteries, aluminum and zinc air batteries have been developed and entered the market, and the research on lithium-air batteries is still basically a blank. Aluminum air battery The aluminum air battery (see Figure 9 for structure) has the following characteristics: Figure 9 Schematic diagram of aluminum air battery structure 1. High energy density: The theoretical energy density of aluminum is 8100Wh/Kg, and the actual energy density of the battery exceeds 350Wh/kg. 2. Easy to operate, long service life: metal electrodes can be mechanically replaced, battery management is simple, and the service life depends only on the working life of the oxygen electrode. 3. The battery has various structures: it can be designed as a primary battery or a secondary battery, and the metal anode can be a plate type, a wedge type or a paste body, and the electrolyte can be circulated or not. 4. Circular economy: The battery consumes aluminum, oxygen and water to form metal oxides. The latter can be reduced by renewable energy such as water, wind energy and solar energy. For ordinary cars, 3kg of aluminum and 5L of water are consumed per 100km, and the recycling cost is less than 10 yuan. 5. Green and environmental protection: non-toxic, no harmful gases, no pollution to the environment. 6. Adequate raw materials: Aluminum is the most abundant metal element on the earth and has a low price. The global aluminum industry's industrial reserves exceed 25 billion tons, which can meet the needs of the automotive industry's electric vehicle power battery. The core technologies of aluminum air battery research include: preparation of aluminum alloy electrode, research on anodic corrosion and passivation; preparation of air diffusion electrode and research on oxygen reduction catalytic material; research on preparation and treatment system of electrolyte, inhibiting anodic corrosion and reducing Polarization, improve battery efficiency; electrolyte circulation system, air circulation guarantee system and battery pack thermal management system; mechanical charging, mechanical replacement of new anode after alloy anode discharge, discharge product and electrolyte centralized regeneration treatment, recycling. According to Tang Yougen, Central South University and China Zhide Group have launched electric vehicle aluminum air batteries with an energy density of more than 350Wh/Kg. The battery has been integrated and the capacity has reached more than 5000Ah, which can enter the market. The practical application cost of aluminum air battery includes: aluminum air battery consumption of 1kg aluminum can produce 3.6~4.8 degree DC, which is equivalent to 1.5~2.0 liters of diesel driving energy. The reduction of 1kg of aluminum consumes 12 degrees of electricity, the grid low-cost electricity cost is about 12x0.30=3.6 yuan, the logistics cost before and after aluminum reduction is 0.3 yuan/1kg, the depreciation and operation cost of the reduction equipment is 0.3 yuan/1kg, and the total cost is 4.2 yuan. The cost of replacing 1 liter of diesel is about 2.1~3.1 yuan, which is more than 50% lower. Zinc air battery The power density of the zinc-air battery developed by Boxin Battery is 101.4W/kg. The current power fuel cell is 90.9W/kg, the former is 11.6% higher than the latter; the zinc-air battery energy density is 218.4Wh/kg, and the electric fuel cell is 197.7. Wh/kg, the former is 10.5% higher than the latter. The zinc-air battery has the characteristics of low carbon and emission reduction: the energy of 3.5 tons of zinc fuel is about the same as that of 1 ton of diesel, and the 2145Kwh grid can produce 1 ton of zinc fuel. In 2010, China will consume 140 million tons of diesel and consume 63 million tons of gasoline. If 50% of them are replaced by zinc fuel, they can reduce 317.78 million tons of CO2, 11.39 million tons of CO, 1.68 million tons of HC, and 1,140,500 tons of NOx. In analyzing aluminum/magnesium air batteries, hydrogen-oxygen fuel cells, and lithium-air batteries, Yang Deqian pointed out that aluminum/magnesium air batteries must solve the following two problems before they can be used in electric vehicles: power density should be increased by 5 times; aluminum/magnesium should be eliminated. Recycling pollution and significantly reducing the energy used in the material preparation process. Hydrogen-oxygen fuel cells have the following problems: the electrolysis production of hydrogen consumes too much energy; the transportation of hydrogen vehicles is small and dangerous, such as pipeline transportation, the leakage can reach 40%; the hydrogen in the hydrogen storage tanks on the vehicle is currently only It accounts for 3~5% of the mass of the tank; there is no catalyst that can really replace platinum. For example, the Mercedes-Benz Citaro oxyhydrogen fuel cell vehicle consumes 17.0 hydrogen per 100 kilometers, and the electricity consumption per kilogram of fuel is 64-72 kWh, which translates to 1001 to 1227 kWh of electricity consumption per 100 kilometers. Therefore, it is necessary to greatly reduce the energy consumption of hydrogen production. Before the above problems were solved, it seems impossible for oxyhydrogen fuel cells to be commercialized. In addition, the United States and Canada have stopped research and development of automotive oxyhydrogen fuel cells. Lithium-air batteries are still in the early stages of research. The problems to be solved include: preventing the chronic leakage of the diaphragm using two electrolytes; increasing the usable temperature of the organic electrolyte; finding a gold and platinum catalyst that can replace the current use; How to prevent the intrusion of water vapor to cause explosion when replacing lithium fuel; how to recycle unused lithium and lithium hydroxide; how to reduce the energy consumption of circulating lithium hydroxide. In summary, he believes that zinc-air battery is not the best battery, but it is the most practical battery.
LED flexible neon light: Replacement for
traditional Neon tube (glass tube) of Structure outline such as roofs, windows, doors, storefronts and carports. Perfect for home and garden lighting decoration.
Easy to install: Waterproof Neon light
strip can use Indoor and Outdoor.
Low power Consumption: Super bright LED
light running with low temperature. All the light spread and completely smooth,
luminous very even.
Flexible & cuttable: Neon light strip
can be shaped, formed or bent in any angle. It can be cut in every marked point
without damaging the rest strips.
Flexible Neon Lights,Led Flexible Neon Lights,Neon Light Fixtures,Flexible Led Neon Strip Light Shenzhen Oleda Technology Co.,Ltd , https://www.baiyangsign.com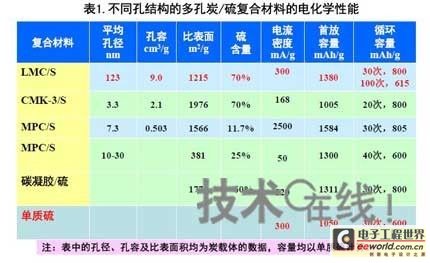
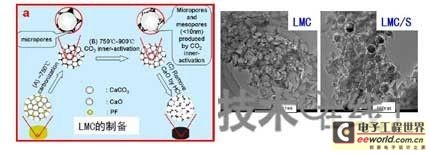
The preparation process of large mesoporous carbon is: using nano CaCO3 as a template, phenolic resin as carbon source, carbonization, activation in CO2, HCL stenciling, and water washing. The surface density was 1215 cm 2 /g, the pore volume was 9.0 cm 3 /g, and the conductivity was 23 S/cm. Then, it was co-heated with sulfur at a high temperature of 300 ° C to prepare an LMC/S material, in which S accounted for 70%. as shown in picture 2.